Abstract
Background Endemic Burkitt lymphoma (eBL), which occurs at a disproportionately high rate among children in Africa, is associated with Plasmodium falciparum (Pf) malaria and Epstein-Barr virus (EBV). Genetic factors, including Human leukocyte antigen (HLA) system, are hypothesized to play a role in eBL by modulating the immune response or susceptibility to associated pathogens. However, the association between HLA and eBL has not been comprehensively evaluated to-date.
Methods We investigated the association between HLA variants and eBL among 800 cases and 3,845 controls aged 0-15 years enrolled in two studies: the Epidemiology of Burkitt lymphoma in East African children and minors (EMBLEM) study conducted in Uganda, Tanzania and Kenya (2010-2016) and the Infections and Childhood Cancer Study conducted in Malawi (2005-2008). HLA alleles from eight classical HLA genes were imputed from genome wide data (Illumina 5 M array) with the SNP2HLA program using an updated multi-ancestry HLA reference panel (n=21,456). We demonstrated accurate imputation at 2-field resolution by comparing the imputed HLA alleles against HLA alleles obtained by sequence-based typing of 600 EMBLEM participants (200 cases and 400 controls) from Uganda. We performed country-specific association tests using generalized linear mixed models (GLMMs), adjusting for sex (genetic confirmed), age, falciparum positivity, and population structure using the top 3 principal components and accounting for genetic relatedness. Odds ratios (OR) were used to estimate the effect size for each HLA variant and significance was assessed by Wald tests. Meta-analyses were performed to obtain summary effects across countries. For 12 HLA alleles with previously reported associations with severe malaria (e.g., HLA-B*53), EBV antibodies (e.g., HLA-DRB1*15:01) or eBL (e.g., HLA-A*02), were hypothesized, apriori, that we would observe associations with eBL mirroring biologic effects of prior associations. Thus, a P value of 0.05 was considered sufficient for statistical significance. For all other associations, we adjusted for multiple testing with P<2.7×10-4 (correction for 187 tested HLA alleles) used to determine statistical significance for classical HLA alleles at 1- and 2-field resolution and P<1.1×10-6 (correction for 46,350 HLA variants) for all other variants in the HLA region. Conditional analyses were performed to identify HLA variants that independently influence eBL susceptibility.
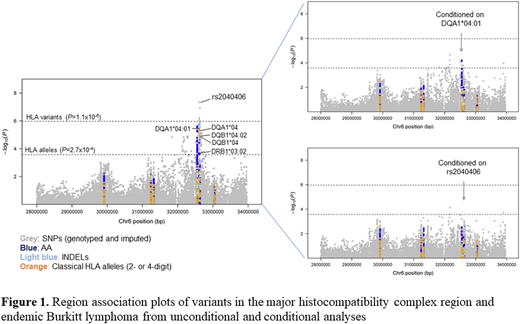
Results We found high concordance rates (>85%) of HLA imputation at all HLA class I and class II alleles of 2-field resolution. The frequency distribution of imputed HLA alleles was similar to that based on sequencing-based HLA typing in a subset of Ugandan participants (Pearson r=0.97). The expected associations between eBL and HLA alleles were only observed for HLA-A*02 and -B*41 based on a nominal p<0.05. More importantly, we found significant associations between eBL and HLA-DQA1*04:01 (adjusted odds ratio [aOR]= 1.61, 95% confidence interval [CI]=1.32 to 1.97, P=3.71×10-6) and SNP rs2040406, located in the HLA-DQA1 region (aOR=1.43 (95%CI=1.26 to 1.63), P=4.62×10-8) (Figure 1). No other HLA alleles or non-allele variants were significantly associated with eBL in conditional analysis on HLA-DQA*04:01 or rs2040406. The novel association with SNP rs2040406 corresponds to a single residue Gln53 within the HLA-DQA1 peptide-binding groove that tracks with rs2040406 (r2 = 0.88) and showed the strongest association among all residues (aOR=1.36, P=2.06×10-6).
Conclusions We present a comprehensive investigation of the associations between eBL and HLA variants utilizing imputed HLA data. Consistent associations were observed for two eBL-based apriori-stipulated hypotheses (HLA-A*02 and-B*41); however, contrary to our expectations, the malaria-based apriori hypotheses were not confirmed. Our agnostic analysis identified new associations between eBL and variants in the HLA-DQB1/DQA1 region, thereby providing new directions to explore the underlying relationship between HLA, immunity and eBL risk. In particular, the association of eBL with rs2040406 was attributed to residue Gln53 within the HLA-DQA1 peptide-binding groove. These associations hint at functional effects, perhaps mediated by stronger binding of EBV glycoproteins.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal